
Double reciprocal 1/V versus 1/[S] lineweaver burk plot for the... Download Scientific Diagram
The Lineweaver-Burk equation is: 1 V 0 = Km V M AX ⋅ [S] + 1 V M AX 1 V 0 = K m V M A X ⋅ [ S] + 1 V M A X. where: 1/V 0 = Inverse Velocity in seconds-liters per mole (s·L/mol), where v0 v 0 is the reaction rate. [S] = Concentration of the substrate in moles per liter. V max = Reaction Rate with excess substrate in units of mols L⋅ s.

LineweaverBurk plots for steady state inhibition of AChE (A) and BChE... Download Scientific
Official Ninja Nerd Website: https://ninjanerd.orgNinja Nerds!In this lecture Professor Zach Murphy will continue our discussion about Michaelis Menten and t.

Inhibition (A, B) LineweaverBurk plot analysis of the... Download Scientific Diagram
Lineweaver-Burk Plot for enzyme inhibition. An official website of the United States government. Here's how you know. The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site. The site is secure..

LineweaverBurk plot of the inhibition of nitric oxide synthase... Download Scientific Diagram
Lineweaver-Burk analysis is one method of linearizing substrate-velocity data so as to determine the kinetic constants Km and Vmax. One creates a secondary, reciprocal plot: 1/velocity vs. 1/[substrate].

LineweaverBurk graph for thiamin. Figure 6. Inhibitor concentration... Download Scientific
Prism will generate a new data table titled "Plot a function". Click and drag this table onto the Lineweaver-Burk graph, then click "Add" Notes • See the list of assumptions of all analyses of enzyme kinetics. • This equation fits exactly the same curve as the equation that fits the turnover number Kcat rather than the Vmax. The product of.

LineweaverBurk plots for the inhibition of hMAOA by compound 2. [S],... Download Scientific
In biochemistry, the Lineweaver-Burk plot (or double reciprocal plot) is a graphical representation of the Michaelis-Menten equation of enzyme kinetics, described by Hans Lineweaver and Dean Burk in 1934. [1]

LineweaverBurk Plot and Reversible Inhibition YouTube
Lineweaver-Burk analysis is one method of linearizing substrate-velocity data so as to determine the kinetic constants Km and Vmax. One creates a secondary, reciprocal plot: 1/velocity vs. 1/ [substrate].
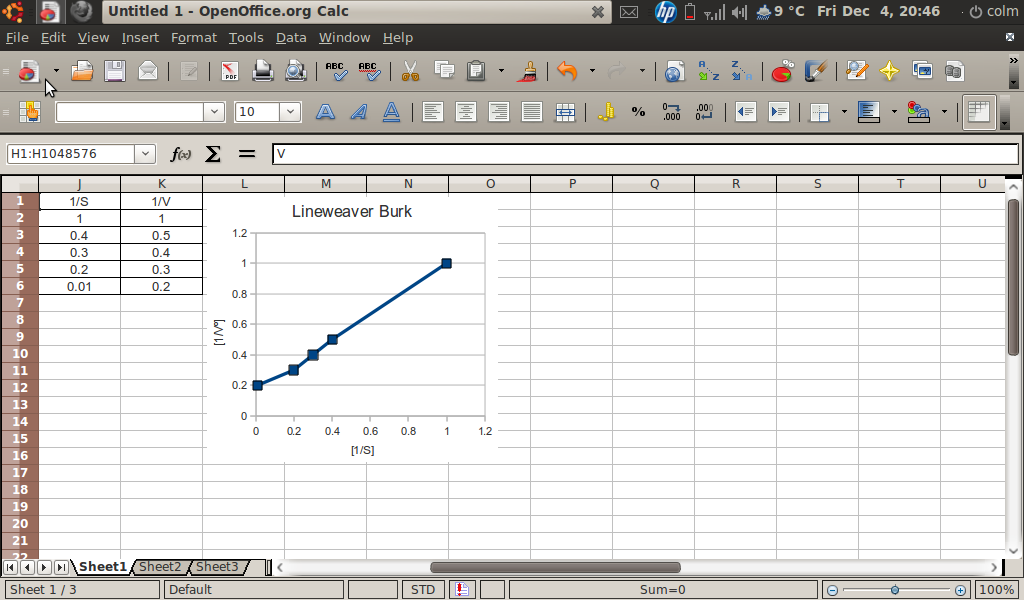
My Meanderings How To Create Lineweaver Burk Graph Openoffice Calc
Figure \(\PageIndex{12}\): Noncompetitive Inhibition: Lineweaver-Burk plots. Move the sliders on this interactive graph below to show changes in K is and K ii affect position on the graph where the lines intersect. Try to change their values to move the intersections of the graphs from the left top quadrant to the x-axis to the left bottom.

Inhibition mechanisms. LineweaverBurk graph for compound (11) (a)... Download Scientific Diagram
Figure 4.9.1: Line-Weaver Burk Plot. For a Lineweaver-Burk, the manipulation is using the reciprocal of the values of both the velocity and the substrate concentration. The inverted values are then plotted on a graph as 1 / V vs. 1 / [ S ]. Because of these inversions, Lineweaver-Burk plots are commonly referred to as 'double-reciprocal' plots.

LineweaverBurk graph in five different substrate (ABTS) concentrations... Download Scientific
The Lineweaver Burk plot is a double reciprocal plot that helps visualize the relationship between the reciprocal of substrate concentration (1/ [S]) and the reciprocal of reaction rate (1/V). By plotting the data in this manner, the Lineweaver Burk plot simplifies the analysis of enzyme kinetics. The purpose of the Lineweaver Burk plot is to.

Pin page
The Lineweaver Burk plot is a graphical representation of enzyme kinetics. The x-axis is the reciprocal of the substrate concentration, or 1 / [S], and the y-axis is the reciprocal of the reaction velocity, or 1 / V. In this way, the Lineweaver Burk plot is often also called a double reciprocal plot.

The LineweaverBurk plot for the ACE inhibition pattern of purified... Download Scientific Diagram
The Lineweaver-Burk plot was widely used to determine important terms in enzyme kinetics, such as \(K_m\) and \(V_{max}\), before the wide availability of powerful computers and non-linear regression software. The y-intercept of such a graph is equivalent to the inverse of \(V_{max}\); the x-intercept of the graph represents \(−1/K_m\).
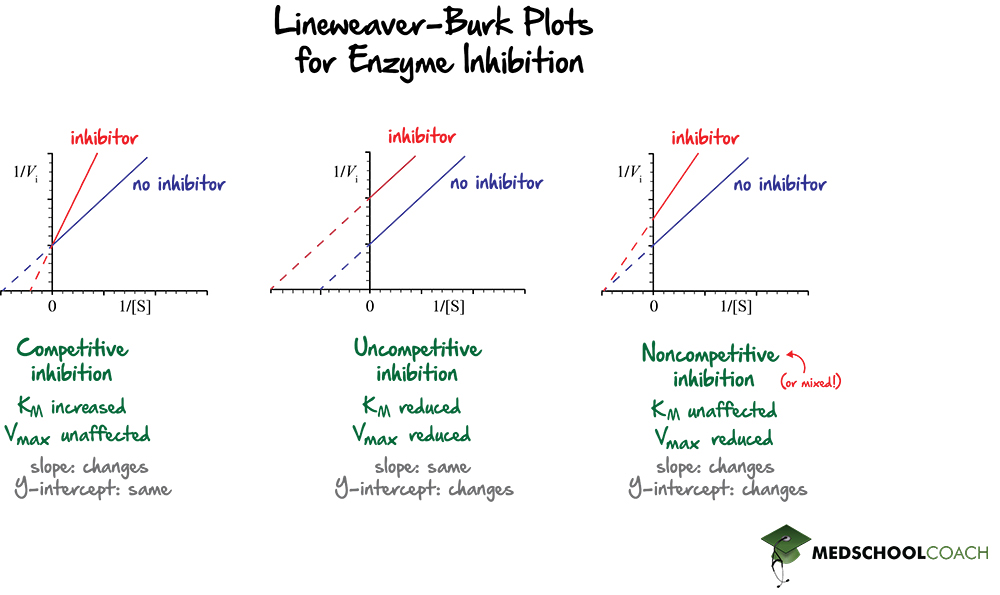
Lineweaver Burk Plots MCAT Biochemistry MedSchoolCoach
Moof's Medical Biochemistry Video Course: http://moof-university.thinkific.com/courses/medical-biochemistry-for-usmle-step-1-examFor Related Practice Problem.

Graph 5 LineweaverBurk Plot scatter chart made by Vanessalim plotly
Note that in the first three inhibition models discussed in this section, the Lineweaver-Burk plots are linear in the presence and absence of inhibitor. This suggests that plots of \(v\) vs. \(S\) in each case would be hyperbolic and conform to the usual form of the Michaelis Menton equation, each with potentially different apparent \(V_m\) and.

Lineweaver Burk double reciprocal plots for the determination of Km... Download Scientific
The Lineweaver-Burk plot (or double reciprocal plot) is a graphical representation of the Lineweaver-Burk equation of enzyme kinetics, described by Hans Lineweaver and Dean Burk in 1934. This plot is a derivation of the Michaelis-Menten equation and is represented as: Table of Contents
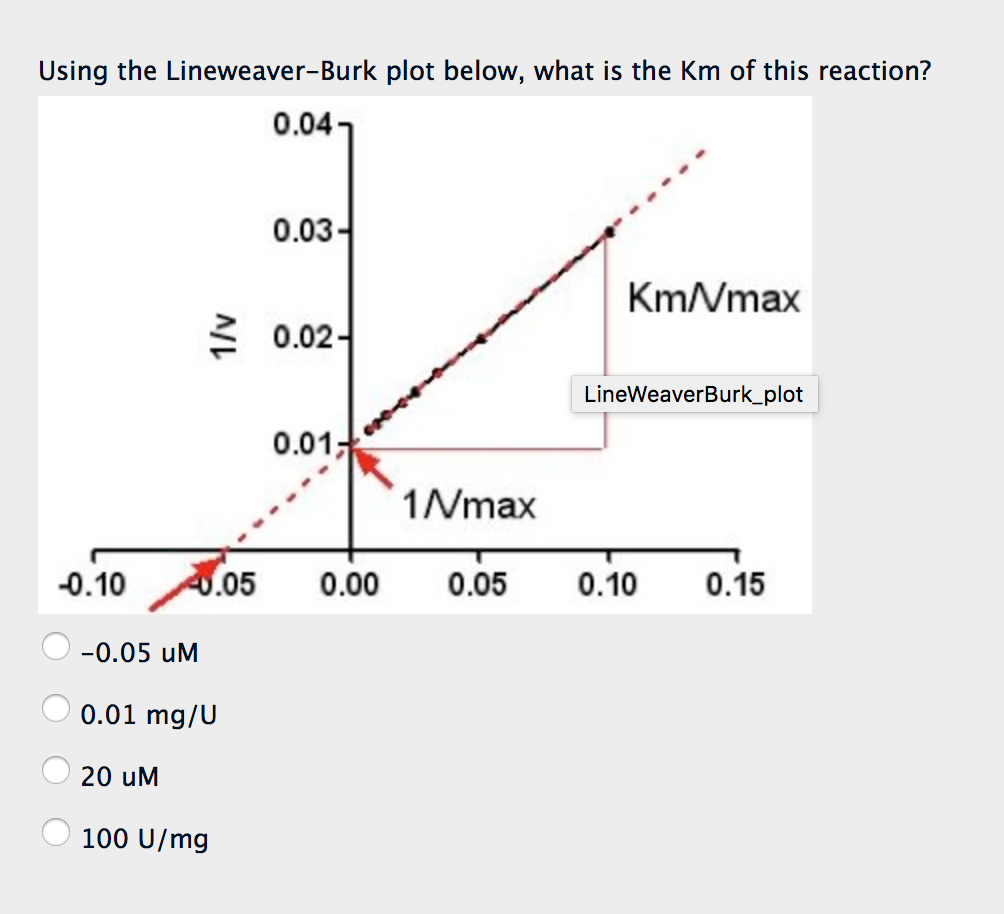
Solved Using the LineweaverBurk plot below, what is the Km
To determine the V max from a Lineweaver-Burk plot you would: A. Multiply the reciprocal of the x-axis intercept by -1. B. Multiply the reciprocal of the y-axis intercept by -1. C. Take the reciprocal of the x-axis intercept. D. Take the reciprocal of the y-axis intercept.